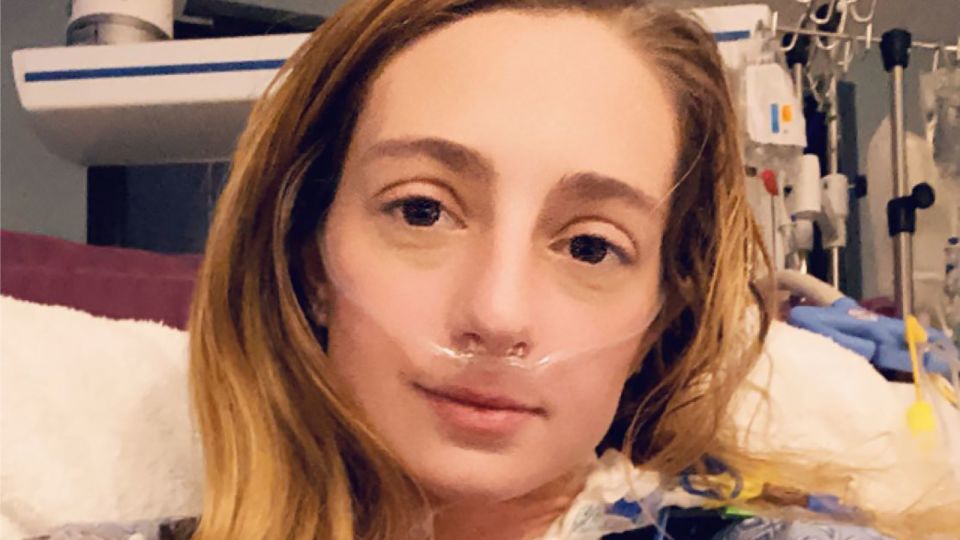
When Katrina Barry was diagnosed with pulmonary arterial hypertension (PAH), a rare and serious condition, she was initially told not to research it online. PAH affects about 500 to 1,000 Americans each year, with a grim prognosis of two to five years to live. However, Barry was offered a lifeline in the form of a first-of-its-kind experimental drug called sotatercept, which targets a growth factor overproduced in people with PAH. Barry eagerly participated in a study to test the medication, which she credits with keeping her alive and allowing her to resume activities she enjoyed before her diagnosis. The drug, now approved by the FDA under the brand name Winrevair, is designed to grab onto and trap proteins called activins that are overproduced in PAH, offering a new treatment approach for patients.
Winrevair is a promising new drug for patients with PAH, offering potential benefits such as improved exercise capacity and possibly slowing disease progression by targeting the underlying biology of the condition. In a clinical trial that included people who took sotatercept or a placebo for six months, those who received the drug were able to walk significantly farther in six minutes, indicating improved exercise capacity. While the drug offers hope for patients with PAH, it is essential to consider potential side effects such as bleeding and dizziness. However, the benefits of the drug outweigh the risks for many patients with limited treatment options.
Katrina Barry’s experience with sotatercept has been life-changing, allowing her to regain her independence and resume activities she once thought impossible. After starting the medication, Barry experienced gradual improvements in her condition, eventually being able to come off continuous oxygen therapy and transition to oral medications for her condition. The drug has enabled her to pursue her goals, such as enrolling in a master’s degree program and participating in activities such as skiing and hiking. Barry sees sotatercept as a “miracle drug” that has brought hope to those with PAH and improved their quality of life significantly.
While sotatercept has shown promising results in clinical trials, there are still many unknowns about the drug, including its long-term effectiveness and potential impact on different patient populations. As the drug becomes available to patients, ongoing research and real-world data will be crucial in determining its overall impact and benefits for a diverse range of individuals with PAH. With a wholesale cost of $14,000 per vial, the medication may be inaccessible to some patients, highlighting the need for insurance coverage and affordability for those who can benefit from this innovative treatment option.
The approval of Winrevair represents a significant advancement in the treatment of PAH, offering hope to patients with this life-threatening condition. While the drug may not be a cure, it provides a new approach to managing PAH and improving outcomes for those affected by the disease. As more patients have access to sotatercept, ongoing research and monitoring will be essential to fully understand the drug’s impact and ensure that it continues to offer benefits to individuals living with PAH. Katrina Barry’s experience serves as a testament to the transformative potential of this medication and its ability to change the lives of those diagnosed with PAH.