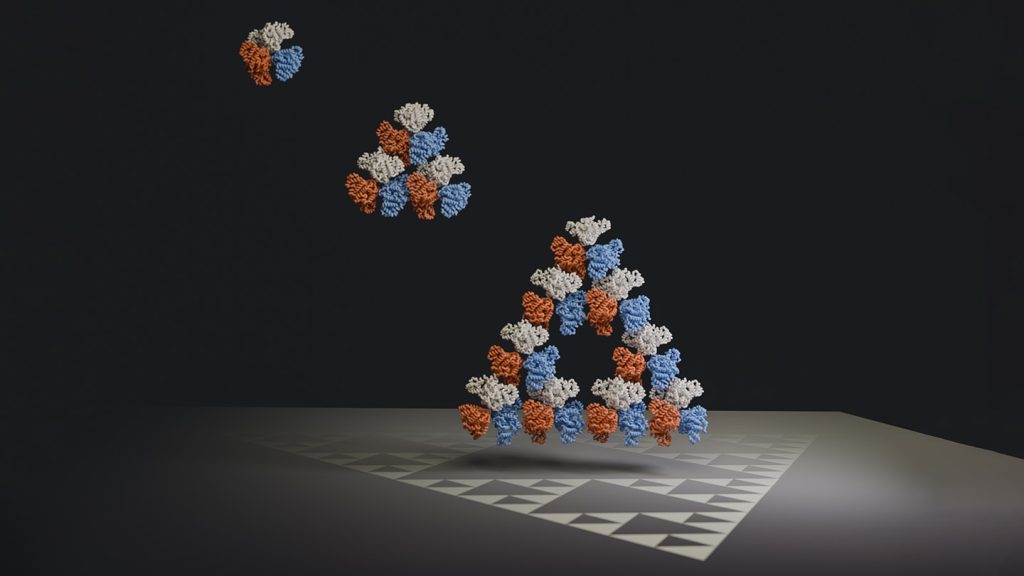
Fractals are complex geometrical patterns found in nature, such as river deltas and tree branches. Regular fractals, which look the same at different scales, have been observed in Romanesco cauliflower whirls. An evolutionary biochemist and his team have now discovered a regular fractal on the molecular level. A protein in the bacterium Synechococcus elongatus forms a Sierpiński triangle, a fractal shape made of interconnected triangles. The protein self-assembles into large triangles composed of smaller triangles, potentially evolving into even more complex structures.
Scientists have previously synthesized molecules that can form regular fractals, but this bacterial protein, citrate synthase, is the first to exhibit such fractal properties in nature. Electron microscope images show the protein arranging into a triangle consisting of smaller triangles. While the purpose of this pattern remains unknown, researchers believe it is likely an evolutionary accident. Accidents, both functional and non-functional, occur in the assembly of proteins over evolutionary timescales. The intricate structures formed by proteins can captivate scientists, leading them to search for meaning in these symmetrical patterns.
Evolutionary biologist Georg Hochberg, from the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, led the research team that discovered the fractal protein in the bacterium. Hochberg notes that complex structures like the Sierpiński triangle can arise and disappear over evolutionary timescales, sometimes serving a purpose and other times not. The symmetry and beauty of these structures can be intriguing to researchers, prompting them to investigate their significance further. Despite the lack of a clear function for the protein’s fractal pattern, the discovery adds to the intriguing diversity of natural structures.
The team’s findings, published in Nature, represent a significant discovery in exploring the intricate world of molecular fractals in nature. The protein’s unique self-assembly into a regular fractal pattern sheds light on the complexity and diversity of biological structures. While the specific function of the Sierpiński triangle formed by citrate synthase remains elusive, the accidental nature of this discovery highlights the complexity of evolutionary processes and the beauty of natural patterns. This research enhances our understanding of how biological molecules can organize themselves into mesmerizing fractal shapes, revealing the hidden beauty of the microscopic world.