The field of neurotechnology is rapidly evolving, with a focus on developing digital dementia screening tools to identify cognitive impairments early in the disease progression. This is crucial, considering that the number of people with Alzheimer’s disease is expected to triple by 2060. However, the challenge lies in finding cost-effective and patient-centered diagnostic tests that can accurately detect mild cognitive impairment and Alzheimer’s disease. The market for dementia screening is complex and rapidly changing, with various technologies and assessments available to healthcare providers.
The current standard of care for evaluating cognitive health involves a range of cognitive assessments such as MMSE, Mini-Cog, GPCOG, MoCA, and SLUMS. Many technology-based brain health tests are emerging, offering interactive screening processes that incorporate AI for scoring analysis. These tools aim to improve the efficiency and accuracy of cognitive screening, especially in outpatient care where underreporting and stigma are common. The healthcare system is in need of better screening tools to find warning signs and prioritize individuals who require a closer examination.
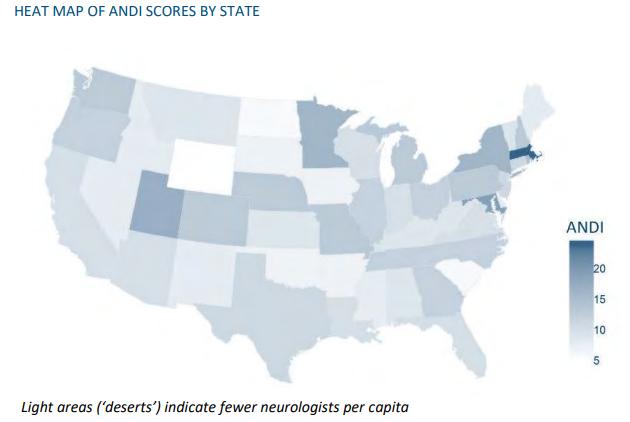
The business pressures in the dementia screening market include challenges related to a shortage of neurologists and primary care physicians, regulatory complexities, reimbursement issues, and the quality of diagnosis. While biomedical science has made significant advancements in identifying biological markers of Alzheimer’s disease through PET scans and cerebrospinal fluid testing, digital tools are also playing a crucial role in the diagnostic landscape. Companies are leveraging AI, games, EHR data, MRI scans, and EEG assessments to improve cognitive screening and diagnosis.
The debate between using blood tests versus digital tests for dementia screening is ongoing, with both approaches having their own advantages. Digital tools offer a cost-effective and scalable solution for early screening, while blood tests promise greater accuracy and reliability. The challenge lies in integrating these innovative technologies into clinical practice and ensuring that they are accessible and affordable for both healthcare providers and patients. The goal is to develop diagnostic workflows that can triage risk and direct appropriate next steps in the diagnostic process.
The implementation, deployment, and validation of early detection tools for Alzheimer’s disease are critical in improving the efficiency and effectiveness of dementia screening. Collaborative projects like AD-RIDDLE are testing real-world economics of dementia screening tools and exploring ways to streamline the screening, diagnosis, and treatment process. Startups, investors, and partners in the neurotech space must navigate the challenges of regulation and reimbursement while diversifying revenue streams and engaging directly with patient and caregiver communities to address the evolving needs of the dementia screening market.
In conclusion, the field of neurotechnology is poised for significant advancements in dementia screening, with a focus on early detection and accurate diagnosis of cognitive impairments. By leveraging innovative digital tools, AI, and biological markers, healthcare providers can improve the efficiency and effectiveness of dementia screening, ultimately leading to better outcomes for patients living with cognitive impairment. Collaborative efforts between industry stakeholders, regulatory bodies, and patient communities are essential in shaping the future of dementia screening and advancing the fight against neurodegenerative diseases.