The Food and Drug Administration warned of fake Botox products causing vision problems and difficulty breathing and swallowing in at least nine states. These counterfeit products were obtained from unlicensed sources and pose serious health risks due to being misbranded, adulterated, counterfeit, contaminated, and ineffective. The FDA, along with the Centers for Disease Control and Prevention, are investigating these cases that have been reported in Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. Authentic Botox made by AbbVie is not linked to any of these illnesses and is considered safe and effective for its approved uses.
Botox is a popular cosmetic treatment that uses a purified form of botulinum toxin to paralyze muscles temporarily, reducing wrinkles. The concern with counterfeit products is that they may spread throughout the body and incapacitate essential muscles, such as those needed for breathing. Botulism caused by fake Botox is considered a medical and public health emergency, and individuals experiencing symptoms should seek immediate medical care. Nineteen women who received what they believed to be real Botox from untrained individuals in non-healthcare settings like homes or spas developed symptoms, mostly seeking injections for cosmetic reasons. While nine patients required hospitalization, all have recovered without the need for a ventilator, and there have been no reported deaths.
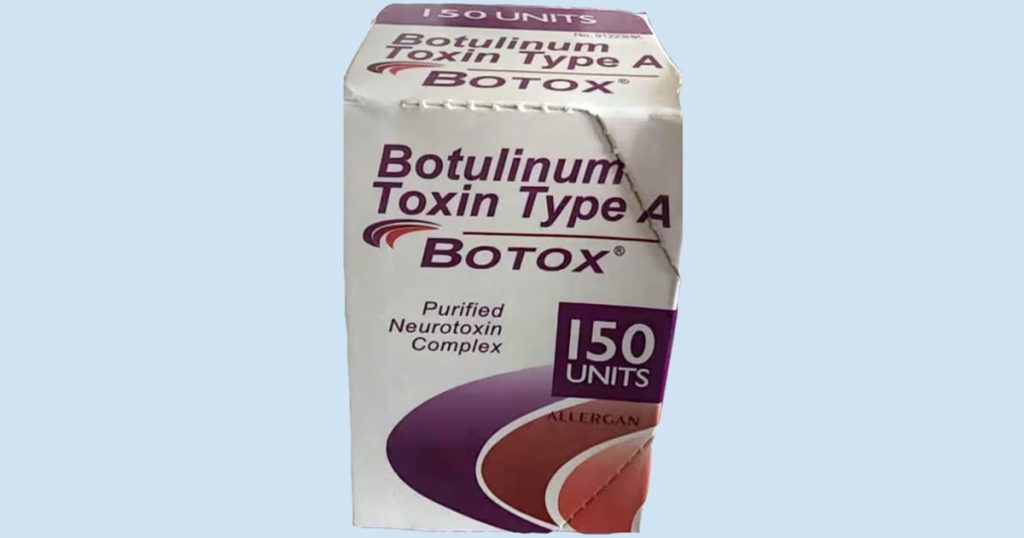
Identifying counterfeit Botox products is crucial for safeguarding against potential risks. The FDA provided several ways to recognize fake Botox, including looking for lot number C3709C3, the active ingredient displayed as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA,” 150-unit doses not made by AbbVie, and non-English language on the outer carton. These signs can help individuals and healthcare providers avoid using fake Botox that may pose severe health threats.
The spread of fake Botox products highlights the importance of seeking treatment from licensed healthcare providers in approved settings to ensure safety and efficacy. The collaboration between the FDA, CDC, and state health departments in investigating and addressing these cases demonstrates a commitment to protecting public health and preventing further harm. Physicians are urged to be aware of the issue and stay informed about potential risks associated with counterfeit Botox products to provide appropriate care to patients who may be affected.
Ensuring the safety and effectiveness of cosmetic treatments like Botox is crucial for protecting individuals from potential health risks. The unauthorized distribution and use of counterfeit Botox products can lead to serious consequences, such as respiratory muscle paralysis, requiring immediate medical attention. By raising awareness about the dangers of fake Botox and providing guidance on identifying authentic products, healthcare authorities aim to prevent further cases of botulism caused by counterfeit injections. Individuals seeking cosmetic treatments should prioritize safety and consult with licensed providers to avoid potential harm from counterfeit products.